2. 广东省建筑设计研究院, 广州 510010
2. Architectural Design and Research Institute of Guangdong Province, Guangzhou 510010, P. R. China
水源水污染日益严重,水华现象频发,对饮用水水质安全造成威胁。水华主要发生在春、秋季且优势种季节性转变明显,蓝藻和硅藻分别是春季和秋季水华的典型优势藻种[1-3]。藻细胞及藻类有机物(AOM)会降低混凝效率、堵塞滤池、引起膜污染并增加消毒副产物的形成[4-6]等。传统水处理工艺主要通过混凝去除藻细胞,常选用铁盐或者铝盐混凝剂[7]。混凝除藻安全性高,不破坏藻细胞释放藻毒素,对多种藻都有去除效果[8]。目前对混凝除藻的研究中,对混凝剂的种类、用量和混凝条件等优化的研究居多[9-11]。混凝剂的水解产物对胶体颗粒产生电性中和或吸附架桥作用生成絮体并聚沉是混凝的基础[12],混凝絮体的粒径、强度和密度等特性对混凝效果尤为关键[8]。目前国内外关于混凝絮体的特性研究多集中在天然有机物(NOM)、无机絮体以及混凝剂投量对絮体特性的影响,而关于藻类混凝机制和混凝絮体特性的研究十分有限[13, 14]。Clasen等[15]最早提出,由于藻细胞释放AOM阻碍混凝剂与细胞表面的电中和作用,藻类絮体生长缓慢。蓝藻细胞可利用液泡调节细胞密度,而具有突起的硅藻会阻碍细胞与絮体直接接触,均加大了处理难度[4, 16]。藻种的类别不同,其形态结构、细胞密度和聚集程度等差异均会影响混凝过程[17, 18]。通过对比研究春、秋季水华优势藻种中不同形态藻类的混凝效果和絮体特性,对优化混凝除藻具有重要意义。
笔者选取蓝藻门和硅藻门中形态、结构差异较大的铜绿微囊藻,针杆藻和水华鱼腥藻为实验藻种。通过研究上述3种藻在铁盐和铝盐混凝剂下的混凝效果和絮体特性差异,探究藻类对混凝的影响机制。
1 材料与方法 1.1 材料与试剂实验藻种均购于中国科学院淡水藻种库,蓝藻(铜绿微囊藻、水华鱼腥藻)和硅藻(针杆藻)分别使用BG11和AGP标准培养基。混凝剂FeCl3(AR,阿拉丁)和Al2(SO4)3 · 18H2O(AR,阿拉丁)储备液现配现用;培养基所用试剂均为分析纯,购自阿拉丁;所有溶液均用超纯水配制。
1.2 实验方法蓝藻采用恒温光照培养箱(GXZ型智能PGX型,宁波东南),参数设定:温度(25.0±1.0)℃,光/暗周期12 h ∶ 12 h,光照强度2 500 Lx。硅藻采用恒温光照培养箱(Bluepard,上海一恒),参数设定:温度(20.0±1.0)℃,光/暗周期14 h ∶ 10 h,光照强度2 000 Lx。所有实验所用藻液均采自对数期,藻细胞浓度为劣5级水华浓度(约108 L-1)。
1.2.1 混凝实验和混凝絮体的形成、破碎及再生实验藻类混凝实验相关实验均在六联搅拌器(ZR4-6,深圳中润)中进行,用超纯水将实验藻种的密度统一稀释至约108 cells/L,pH统一调至7.0。混凝实验搅拌程序:快搅1 min (200 r/min,G=452 s-1);慢搅15 min(50 r/min,G=84 s-1);静置15 min。混凝絮体形成、破碎及再生实验搅拌程序:快搅1 min (200 r/min,G=452 s-1);慢搅10 min (50 r/min,G=84 s-1);破碎搅2 min(200 r/min,G=452 s-1);再絮凝搅12 min (50 r/min,G=84 s-1)。
1.2.2 分析方法叶绿素a含量采用反复冻融-丙酮萃取-分光光度法[19]测定;浊度采用浊度仪(2100Q,美国哈希)测定;分形维数值采用计盒维数算法通过Matlab形成二值图,编程计算得到[20];絮体Zeta电位采用Zeta电位仪(Nano ZS90,英国)测定;絮体粒径(d50)采用马尔文激光粒度仪(Mastersizer 2000,英国)测定。测定所有实验结果均为3组平行样均值。
1.2.3 絮体生长速度、强度以及恢复因子的计算絮体生长速度=絮体生长粒径变化/絮体生长时间[21]。絮体的强度因子Sf和恢复因子Rf分别定义如下[22, 23]:
| $ \begin{gathered} \mathrm{S}_{\mathrm{f}}=\frac{d_{2}}{d_{1}} \times 100, \end{gathered} $ | (1) |
| $ R_{\mathrm{f}}=\frac{\left(d_{3}-d_{2}\right)}{\left(d_{1}-d_{2}\right)} \times 100, $ | (2) |
式中:d1为絮体破碎前的粒径;d2为絮体破碎后的粒径;d3为絮体破碎再生后的粒径。
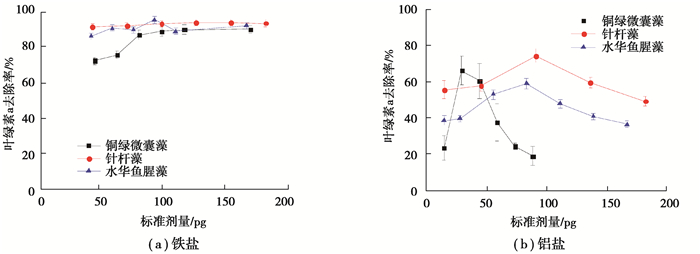
2 结果与讨论 2.1 不同形态藻类对混凝效果的影响各藻种在不同投加量铁盐和铝盐下的叶绿素a和浊度去除率分别如图 1、2所示。本文中的实验及相关论述中,混凝剂的投加量均指单位藻细胞的混凝剂投加量。由图 1、2可知,在铁盐和铝盐的实验投加量范围内(铁盐:44~180 pg;铝盐:15~180 pg),3种藻的叶绿素a和浊度去除率都随混凝剂投加量增加呈先上升后稳定或下降趋势。上述现象可用吸附-电中和机理解释为:当混凝剂超过一定投量时,带负电的藻细胞及AOM表面吸附带正电的铁或铝的水解聚合阳离子,电中和作用使得藻细胞表面由负电性变成了正电性,导致藻细胞表面电性反转并重新稳定[24]。
|
图 1 各藻种在铁盐和铝盐不同投加量下混凝的叶绿素a去除率 Fig. 1 Chlorophyll-a removal rate of different algae species under different dosages of ferric and aluminum salts |
|
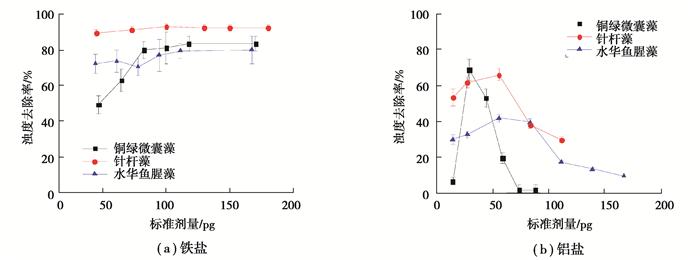
图 2 各藻种在铁盐和铝盐不同投加量下混凝的浊度去除率 Fig. 2 Turbidity removal rate of different algae species under different dosages of ferric and aluminum salts |
值得注意的是:当铁盐投加量超过100 pg时,铜绿微囊藻的叶绿素a和浊度的去除率均超过80%;针杆藻的分别高达90%和89%;水华鱼腥藻的则分别超过86%和71%。3种藻的铁盐混凝效果:针杆藻整体的混凝效果最好;在铁盐低投加量(< 120 pg)时,铜绿微囊藻的混凝效果最差;在铁盐高投加量时,铜绿微囊藻和水华鱼腥藻的混凝效果接近。在铝盐的实验投加量范围内,铜绿微囊藻的叶绿素a和浊度去除率分别在18%~66%和2%~69%;针杆藻的叶绿素a和浊度去除率分别在49%~74%和30%~66%;水华鱼腥藻的叶绿素a和浊度去除率分别在36%~59%和9%~42%。总体而言,铁盐对3种藻的混凝沉淀去除效果优于铝盐。这主要归因于铁盐的水解产物吸附架桥能力较强,对藻细胞吸附作用强,生成絮体的体积和比重大、沉降快[25];铝盐生成絮体细小疏松、沉降慢,同时铝盐更容易与藻细胞的官能团螯合[26]。此外,在铁盐、铝盐各自的实验投加量范围内,3种藻在采用铁盐混凝时的叶绿素a和浊度最佳去除率对应的铁盐投加量,均大于采用铝盐混凝时叶绿素a和浊度最佳去除率对应的铝盐投加量。
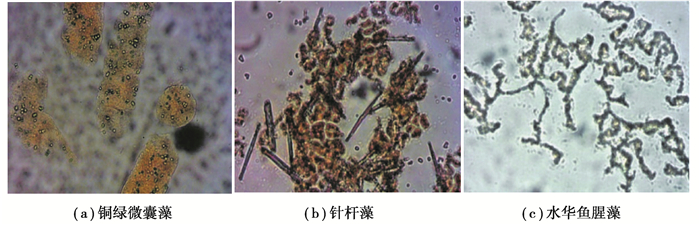
2.2 不同形态藻类对混凝絮体特性的影响 2.2.1 絮体的表观特性及分形维数各藻种细胞混凝絮体的形态结构见图 3。当采用铁盐混凝剂时,铜绿微囊藻细胞小,分散在絮体中,絮体的特征长度大[27],絮体密度低;针杆藻本身尺寸大,与包裹的铁盐絮体聚集成更紧密的大絮团,另外其硅质外壳比重较大故容易下沉;水华鱼腥藻混凝絮体悬挂在藻丝上,结构较疏松比重小,不易下沉。
|
图 3 不同藻种细胞铁盐混凝絮体的形态 Fig. 3 Morphology of ferric salt coagulated flocs of different algal cells |
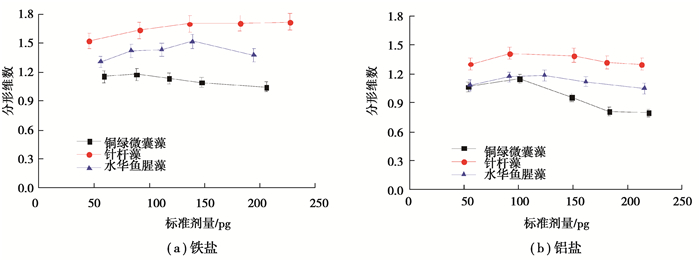
絮体的分形维数主要反映絮体的密实程度,其值越大表示絮体越密实[28],沉降效果越好。由图 4可知,随着铁盐、铝盐投加量的增加(45~230 pg),3种藻的分形维数均呈先上升后下降或稳定的趋势,与2.1节中3种藻混凝效果整体的变化趋势基本一致。当采用铁盐作为混凝剂时,铜绿微囊藻的分形维数值在铁盐投加量为88 pg时最大,为1.17;针杆藻的分形维数值在铁盐投加量为230 pg时最大,为1.72;水华鱼腥藻的分形维数值在铁盐投加量为140 pg/时最大,为1.51。采用铝盐作为混凝剂时,3种藻细胞混凝絮体的分形维数值均小于对应铁盐混凝絮体的分形维数值,表明铁盐絮体比铝盐絮体更加密实,这与上述2.1节中铁盐对3种藻的混凝效果优于铝盐这一结论相符。在铁盐、铝盐不同投加量下,3种藻的分形维数值:针杆藻最大,铜绿微囊藻最小,进一步表明了藻种形态对絮体结构的影响。
|
图 4 各藻种在铁盐和铝盐不同投加量下混凝的分形维数值 Fig. 4 The fractal dimension of different algae flocs under different dosage of ferric and aluminum salts |
当采用铁盐和铝盐混凝剂时,各藻种混凝絮体生长-破碎-再生过程中絮体粒径(d50)的变化如图 5所示,总结絮体的生长速度、d50最大值、强度因子和恢复因子见表 1。
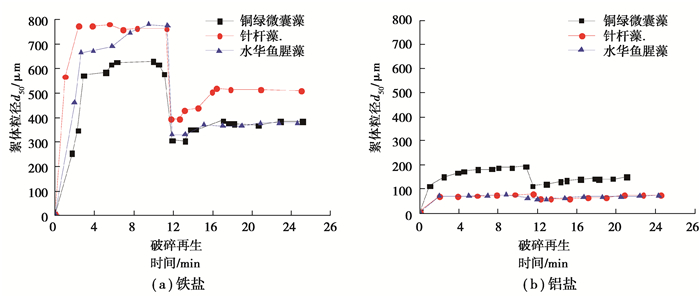
|
图 5 各藻种铁盐和铝盐混凝絮体的生长-破碎-再生过程 Fig. 5 The growth-breaking-regeneration process of algae coagulated flocs with ferric and aluminum salts |
| 表 1 各藻种混凝絮体的生长速度、d50最大值、强度因子和恢复因子 Table 1 Growth rates, maximum d50, intensity factors and recovery factors of different algal coagulated flocs |
絮体粒径能反映细胞沉降性能,采用铁盐混凝剂的3种藻絮体的生长速度和d50远大于采用铝盐混凝剂的对应值。当采用铁盐混凝剂时,3种藻混凝絮体d50的最大值在632~777 μm,远大于采用铝盐混凝剂对应的76~194 μm,梁娟等[29]在对原水混凝絮体的研究中得出了类似结论。当采用铁盐作为混凝剂时,铜绿微囊藻絮体的生长速度和d50最大值均小于针杆藻和水华鱼腥藻的对应值,这与藻细胞体积有关。针杆藻细胞呈壳面线形,细胞宽4~6 μm、长100~300 μm[30];水华鱼腥藻细胞宽4~8 μm、长6~8 μm;而铜绿微囊藻细胞直径只有3~7 μm。Gonzalez-Torres[7]发现含藻混凝絮体比天然有机物质形成的混凝絮体(535~596 μm[31])和高岭土絮体都大,这归因于藻细胞本身的体积较大。
絮体强度是衡量混凝后续固液分离效果的重要指标。水厂实际运行中通过堰、壁板等高剪切区域时,絮体必须具备足够大的强度来抵抗剪切力,絮体强度越大即对抗剪切破碎的能力越强。当采用铁盐混凝剂时,3种藻絮体的强度因子在45.77%~53.75%,小于采用铝盐混凝剂时对应的62.01%~79.49%。因为粒径大的絮体在快速搅拌时易破裂,粒径小的絮体抵抗剪切的能力反而强,这与Zhao等[32]的研究结论一致。
恢复因子表示絮体的形态受到剪切破坏后的再聚集能力,絮体恢复因子越大则越容易重组成较大的二级絮体[23]。当采用铁盐混凝剂时,3种藻絮体的恢复因子在11.04%~26.54%,小于采用铝盐混凝剂对应的35.94%%~96.97%。这是因为铝盐混凝絮体在破碎前粒径较小,主要通过电性中和产生范德华力和静电引力,絮体破碎后容易再生。当采用铁盐混凝剂时,水华鱼腥藻所形成絮体的恢复因子仅为11.04%,明显小于针杆藻和铜绿微囊藻絮体。究其原因,水华鱼腥藻的丝状结构使其絮体更容易发生网捕卷扫和架桥连接,絮体间化学键连接增强,而这部分化学键在断裂后无法重新形成[33],导致再生能力减弱。而当采用铝盐混凝剂时,水华鱼腥藻所形成絮体的恢复因子反而显著高于针杆藻和铜绿微囊藻絮体,同样可归因于水华鱼腥藻絮体破碎前粒径小,相对于而言更容易恢复。
2.2.3 Zeta电位Zeta电位反映溶液中悬浮物和胶体的稳定性,电负性越大表示斥力越大,越难絮凝。从图 6可知3种藻的初始电位值为:针杆藻(-11.7 V)>水华鱼腥藻(-15.2 V)>铜绿微囊藻(-31.1 V)。由图 7中3种藻絮体Zeta电位随单位藻细胞混凝剂投加量的变化情况可知,随着混凝剂投加量增加(铁盐:15~570 pg;铝盐:15~270 pg),3种藻絮体的Zeta电位由负变正。前已述及,当混凝剂投加过量时,带负电的藻细胞及AOM表面吸附带正电的铁或铝的水解聚合阳离子,电中和作用使得藻细胞表面由负电性变成了正电性,导致絮体表面电负性降低[34]。
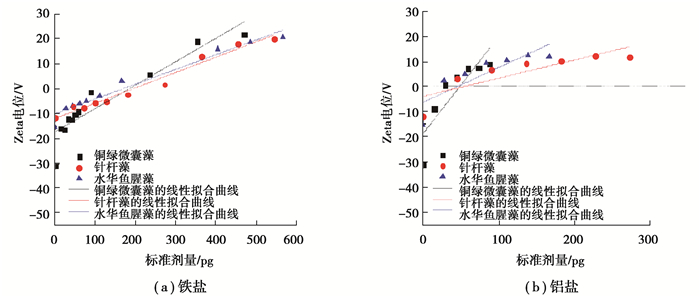
|
图 6 3种藻混凝絮体的Zeta电位随混凝剂投加量的变化情况 Fig. 6 The Zeta potential of different algal coagulated flocs changs under dosage of coagulants |
当溶液处于等电点时,颗粒间无斥力,最容易脱稳促进藻细胞的聚沉。由图 6可知,3种藻絮体到达等电点的铁盐混凝剂投加量大于铝盐,结合2.1节中3种藻最佳去除率对应的铁盐投加量均大于铝盐投加量这一结论,表明Zeta电位可用于分析藻类混凝时最佳去除率对应的投加量。随混凝剂投加量增加,絮体Zeta电位由负变正的快慢可用来分析絮体的电中和能力[35],因此对3种藻絮体Zeta电位随混凝剂投加量的变化情况进行线性拟合,拟合结果见表 2。由表 2可知,铁盐和铝盐混凝剂对铜绿微囊藻的电中和作用最强,对针杆藻的电中和作用最弱,与藻种的混凝效果结论不一致。这种现象说明,3种藻并非都以电性中和为主导作用。铜绿微囊藻的混凝主要以电性中和为主,而吸附架桥和网捕卷扫机制则可能对不规则丝状的水华鱼腥藻和密度大的针杆藻的絮凝作用更重要。邬艳等[36]的研究认为,以网捕卷扫为主导作用形成的絮体比以电性中和为主导形成的絮体更加松散,分形维数值更小。结合上述2.2.1节中,针杆藻和水华鱼腥藻形成的絮体比铜绿微囊藻絮体更加密实,分形维数值也更大,这种差异可能是藻细胞其他性质(AOM和表面官能团等)影响的结果。
| 表 2 3种藻絮体的Zeta电位与混凝剂投加量的线性拟合结果 Table 2 The linear fitting of Zeta potential of different algal coagulated flocs with dosage of coagulants |
1) 总体而言,铁盐对3种藻的混凝沉淀去除效果优于铝盐。3种藻在不同投加量铁盐和铝盐混凝下的絮体特性:铁盐絮体的分形维数值比铝盐絮体的更大;铁盐絮体的生长速度和d50也远大于铝盐。
2) 3种藻在铁盐和铝盐各自达到最佳混凝效果时的混凝剂投加量:铁盐>铝盐,同时3种藻絮体到达等电点的铁盐混凝剂投加量大于铝盐投加量,表明藻絮体Zeta电位可用于分析藻类混凝时最佳去除率对应的投加量。
3) 当采用铁盐混凝剂时,铜绿微囊藻整体的混凝效果最差,叶绿素a和浊度去除率分别在72%~90%和49%~83%;水华鱼腥藻其次,叶绿素a和浊度去除率分别大于86%和71%;针杆藻最好,叶绿素a和浊度的去除率均接近90%。
4) 当采用铁盐混凝剂时,3种藻絮体的形态结构主要取决于藻细胞本身的形态结构。针杆藻铁盐絮体的分形维数值最大,铜绿微囊藻的最小,进一步表明了藻种形态对混凝絮体结构的影响。铜绿微囊藻铁盐絮体d50的最大值为632 μm,小于针杆藻的765 μm和水华鱼腥藻的777 μm,主要由藻细胞体积决定。针杆藻铁盐絮体的恢复因子最大(26.54%),水华鱼腥藻的恢复因子最小(为11.04%),因为水华鱼腥藻在网捕卷扫和架桥连接作用下形成絮体的化学键断裂后无法重新形成。铜绿微囊藻以电性中和混凝机制为主,吸附架桥和网捕卷扫机制则可能对不规则丝状的水华鱼腥藻和密度大的针杆藻的絮凝作用更重要。
| [1] |
黄亚男, 纪道斌, 龙良红, 等. 三峡库区典型支流春季特征及其水华优势种差异分析[J]. 长江流域资源与环境, 2017, 26(3): 461-470. Huang Y N, Ji D B, Long L H, et al. The variance analysis of characteristics and blooms of the typical tributaries of the Three Gorges reservoir in spring[J]. Resources and Environment in the Yangtze Basin, 2017, 26(3): 461-470. (in Chinese) |
| [2] |
Li W, Qin B Q. Dynamics of spatiotemporal heterogeneity of cyanobacterial blooms in large eutrophic Lake Taihu, China[J]. Hydrobiologia, 2019, 833(1): 81-93. DOI:10.1007/s10750-019-3883-1 |
| [3] |
任加国, 贾海斌, 焦立新, 等. 滇池大气沉降氮磷形态特征及其入湖负荷贡献[J]. 环境科学, 2019, 40(2): 582-589. Ren J G, Jia H B, Jiao L X, et al. Characteristics of nitrogen and phosphorus formation in atmospheric deposition in Dianchi Lake and their contributions to lake loading[J]. Environmental Science, 2019, 40(2): 582-589. (in Chinese) |
| [4] |
崔福义, 马华. 水源水中藻的危害与饮用水除藻技术[J]. 给水排水, 2011, 37(6): 1, 103. Chui F Y, Ma H. The harm of algae in water source and the technology of algae removal in drinking water[J]. Water & Wastewater Engineering, 2011, 37(6): 1, 103. (in Chinese) |
| [5] |
Wang H, Park M, Liang H, et al. Reducing ultrafiltration membrane fouling during potable water reuse using pre-ozonation[J]. Water Research, 2017, 125: 42-51. DOI:10.1016/j.watres.2017.08.030 |
| [6] |
Goslan E H, Seigle C, Purcell D, et al. Carbonaceous and nitrogenous disinfection by-product formation from algal organic matter[J]. Chemosphere, 2017, 170: 1-9. DOI:10.1016/j.chemosphere.2016.11.148 |
| [7] |
Gonzalez-Torres A, Putnam J, Jefferson B, et al. Examination of the physical properties of Microcystis aeruginosa flocs produced on coagulation with metal salts[J]. Water Research, 2014, 60: 197-209. DOI:10.1016/j.watres.2014.04.046 |
| [8] |
Nan J, Wang Z B, Yao M, et al. Characterization of re-grown floc size and structure: effect of mixing conditions during floc growth, breakage and re-growth process[J]. Environmental Science and Pollution Research, 2016, 23(23): 23750-23757. DOI:10.1007/s11356-016-7628-7 |
| [9] |
Xu J, Zhao Y X, Gao B Y, et al. Enhanced algae removal by Ti-based coagulant: comparison with conventional Al-and Fe-based coagulants[J]. Environmental Science and Pollution Research, 2018, 25(13): 13147-13158. DOI:10.1007/s11356-018-1482-8 |
| [10] |
Lv L, Zhang X D, Qiao J L. Flocculation of low algae concentration water using polydiallyldimethylammonium chloride coupled with polysilicate aluminum ferrite[J]. Environmental Technology, 2018, 39(1): 83-90. DOI:10.1080/09593330.2017.1296028 |
| [11] |
Chen Y Q, Xie P C, Wang Z P, et al. UV/persulfate preoxidation to improve coagulation efficiency of Microcystis aeruginosa[J]. Journal of Hazardous Materials, 2017, 322: 508-515. DOI:10.1016/j.jhazmat.2016.10.017 |
| [12] |
Chekli L, Eripret C, Park S H, et al. Coagulation performance and floc characteristics of polytitanium tetrachloride (PTC) compared with titanium tetrachloride (TiCl4) and ferric chloride (FeCl3) in algal turbid water[J]. Separation and Purification Technology, 2017, 175: 99-106. DOI:10.1016/j.seppur.2016.11.019 |
| [13] |
Wang N, Li X, Yang Y L, et al. Combined process of visible light irradiation photocatalysis-coagulation enhances natural organic matter removal: Optimization of influencing factors and mechanism[J]. Chemical Engineering Journal, 2019, 374: 748-759. DOI:10.1016/j.cej.2019.05.233 |
| [14] |
Saxena K, Brighu U, Choudhary A. Coagulation of humic acid and kaolin at alkaline pH: complex mechanisms and effect of fluctuating organics and turbidity[J]. Journal of Water Process Engineering, 2019, 31: 100875. DOI:10.1016/j.jwpe.2019.100875 |
| [15] |
Clasen J, Mischke U, Drikas M, et al. An improved method for detecting electrophoretic mobility of algae during the destabilisation process of flocculation: flocculant demand of different species and the impact of DOC[J]. Journal of Water Supply: Research and Technology-Aqua, 2000, 49(2): 89-101. DOI:10.2166/aqua.2000.0008 |
| [16] |
Reynolds C S. Growth, gas vacuolation and buoyancy in a natural population of a planktonic blue-green alga[J]. Freshwater Biology, 1972, 2(2): 87-106. DOI:10.1111/j.1365-2427.1972.tb00364.x |
| [17] |
Lama S, Muylaert K, Karki T B, et al. Flocculation properties of several microalgae and a cyanobacterium species during ferric chloride, chitosan and alkaline flocculation[J]. Bioresource Technology, 2016, 220: 464-470. DOI:10.1016/j.biortech.2016.08.080 |
| [18] |
Gonzalez-Torres A, Pivokonsky M, Henderson R K. The impact of cell morphology and algal organic matter on algal floc properties[J]. Water Research, 2019, 163: 114887. DOI:10.1016/j.watres.2019.114887 |
| [19] |
张亚晴. 掺硼金刚石薄膜电极电化学抑制和去除藻类的效果及机理研究[D]. 重庆: 重庆大学, 2017. Zhang Y Q. Study on the effect and mechanism of the inhibition and removal of algae by electrochemical oxidation using boron-doped diamond electrode[D]. Chongqing: Chongqing University, 2017. (in Chinese) |
| [20] |
陈威. 絮凝搅拌能耗分布优化模式及其实验验证[D]. 武汉: 华中科技大学, 2011. Chen W. Optimal model of stir energy distribution for flocculation and its experimental verification[D]. Wuhan: Huazhong University of Science and Technology, 2011. (in Chinese) |
| [21] |
Wang Y, Gao B Y, Xu X M, et al. Characterization of floc size, strength and structure in various aluminum coagulants treatment[J]. Journal of Colloid and Interface Science, 2009, 332(2): 354-359. DOI:10.1016/j.jcis.2009.01.002 |
| [22] |
Li T, Zhu Z, Wang D S, et al. The strength and fractal dimension characteristics of alum-kaolin flocs[J]. International Journal of Mineral Processing, 2007, 82(1): 23-29. DOI:10.1016/j.minpro.2006.09.012 |
| [23] |
俞文正. 混凝絮体破碎再絮凝机理研究及对超滤膜污染的影响[D]. 哈尔滨: 哈尔滨工业大学, 2010. Yu W Z. Study on flocbreakage and re-growth and its effect on ultrafiltration membrane fouling[D]. Harbin: Harbin Institute of Technology, 2010. (in Chinese) |
| [24] |
李圭白, 张杰. 水质工程学[M]. 北京: 中国建筑工业出版社, 2013, 上册. Li G B, Zhang J. Water quality engineering[M]. Beijing: China Architecture & Building Press, 2013, Volume 1. (in Chinese) |
| [25] |
Jarvis P, Banks J, Molinder R, et al. Processes for enhanced NOM removal: beyond Fe and Al coagulation[J]. Water Supply, 2008, 8(6): 709-716. DOI:10.2166/ws.2008.155 |
| [26] |
Vandamme D, Foubert I, Fraeye I, et al. Influence of organic matter generated by Chlorella vulgaris on five different modes of flocculation[J]. Bioresource Technology, 2012, 124: 508-511. DOI:10.1016/j.biortech.2012.08.121 |
| [27] |
Jiang Q, Logan B E. Fractal dimensions of aggregates determined from steady-state size distributions[J]. Environmental Science & Technology, 1991, 25(12): 2031-2038. |
| [28] |
李冬梅, 梅胜, 谭万春, 等. 黄河泥沙架桥絮凝体分形结构的动态演变研究[J]. 给水排水, 2004, 30(11): 1-5. Li D M, Mei S, Tan W C, et al. Study on dynamic evolution of fractal structure of bridging flocculated sand aggregates in Yellow River[J]. Water & Wastewater Engineering, 2004, 30(11): 1-5. (in Chinese) |
| [29] |
梁娟, 杨青, 丁然, 等. 混凝剂投加量对水质及絮体尺寸变化特性的影响[J]. 给水排水, 2012, 38(S1): 5-9. Liang J, Yang Q, Ding R, et al. The relationship between coagulant dose and water quality and floc size characteristics[J]. Water & Wastewater Engineering, 2012, 38(S1): 5-9. (in Chinese) |
| [30] |
蒋绍阶, 蒋晖, 向平, 等. 强化混凝去除尖针杆藻[J]. 环境工程学报, 2013, 7(09): 3312-3318. Jiang S J, Jiang H, Xiang P, et al. Reinforced coagulation to remove needle needle algae[J]. Chinese Journal of Environmental Engineering, 2013, 7(9): 3312-3318. (in Chinese) |
| [31] |
Sharp E L, Jarvis P, Parsons S A, et al. Impact of fractional character on the coagulation of NOM[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2006, 286(1/2/3): 104-111. |
| [32] |
Zhao Y X, Wang Y, Gao B Y, et al. Coagulation performance evaluation of sodium alginate used as coagulant aid with aluminum sulfate, iron chloride and titanium tetrachloride[J]. Desalination, 2012, 299: 79-88. DOI:10.1016/j.desal.2012.05.026 |
| [33] |
Wang B, Shui Y Y, He M, et al. Comparison of flocs characteristics using before and after composite coagulants under different coagulation mechanisms[J]. Biochemical Engineering Journal, 2017, 121: 107-117. DOI:10.1016/j.bej.2017.01.020 |
| [34] |
Jiang J Q, Graham N. Removal of algae and air halomethane (THM) precursors by coagulation[J]. Water Treatment, 1992, 7: 155-168. |
| [35] |
Ordaz-Diaz L A, Valle-Cervantes S, Rodriguez-Rosales J, et al. Zeta potential as a tool to evaluate the optimum performance of a coagulation-flocculation process for wastewater internal treatment for recirculation in the pulp and paper process[J]. Bioresources, 2017, 12(3): 5953-5969. |
| [36] |
Ordaz-Díaz L A, Valle-Cervantes S, Rodríguez-Rosales J, et al. Zeta potential as a tool to evaluate the optimum performance of a coagulation-flocculation process for wastewater internal treatment for recirculation in the pulp and paper process[J]. BioResources, 2017, 12(3): 5953-5969. |
| [37] |
邬艳, 杨艳玲, 李星, 等. 三种常见混凝机理为主导条件下絮体特性研究[J]. 中国环境科学, 2014, 34(1): 150-155. Wu Y, Yang Y L, Li X, et al. Study on flocs characteristics under three common dominant coagulation mechanisms[J]. China Environmental Science, 2014, 34(1): 150-155. (in Chinese) |
 2022, Vol. 45
2022, Vol. 45


